How to Determine Limiting Reactant
Get the number of moles of reacting substances from the given amounts of reactants. The reactant the produces the least amount of product is the limiting reactant.

How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube
Calculate the number of moles H 2 3022400.

. Calculate the number of moles of. To determine the limiting reactant calculate the amount of product formed by each reactant. We will work out a couple examples together and at the end of the.
Follow us on Twitter Viking_Science. 3H2 N2 --- 2NH3. It is the limiting reactant.
Formula to calculate limiting reactant. How To Find Limiting Reactant. The limiting reactant can also be identified by comparing the quantity of products formed from each of the reactant.
Write the balanced equation. The next step involves. Explains limiting reactant and how to determine which reactant in a chemical reaction is limiting.
Calculate the number of moles H 2 given volume molar volume. Click Create Assignment to assign this modality to your LMS. It will be completely used up in the course of the.
Balance the chemical equation. Begin with a balanced chemical equation with given amounts of both reactants. Parrott teaches you how to determine the limiting reactant in a chemical reaction.
In this video we will learn how to determine the limiting reactant of a chemical reaction. One of given amounts will be the limiting reagent. The reagent produces lesser number of product with comparing to the.
Those are called the excess reactants. The limiting reagent depends on the mole ratio and not on the masses of the reactants present. To determine the limiting reactant following steps should be taken.
Divide the actual number of moles of each reactant by its. Learn how to identify the limiting reactant in a chemical reaction and use this information to calculate the theoretical and percent yields for the reaction. One of the highlighted hurdles in limiting reactant problems is the unbalanced chemical.
It is also essential to. We have a new and improved. So in the above problem o 2 is the limiting reactant because limiting reactant reactant that produces least ml of product.
Once you find the moles only convert one of them to the moles of the. Determine the number of moles of each reactant. Identify the Limiting Reactant Step 1.
There are several ways to find the limiting reagent. There are only 025 moles of HCl instead of 03 moles so the HCl will run out first. How to Find the Limiting Reactant.
Equate the reactant coefficients as ratios. Consider the following reaction for the formation of ammonia. To determine which reactant is the limiting reactant first determine how much product would be formed by each reactant if all the reactant was consumed.
One key is to always start with a balanced chemical reaction. EqSiF_4 g 2 H_2O rightarrow SiO_2 s 4HF g eq Step 2. Now use the moles of the limiting reactant to calculate the mass of the.
The way I calculate the limiting reactant is by first finding the amount of moles are in the reactants given. Divide by the coefficients of the hydrogen.
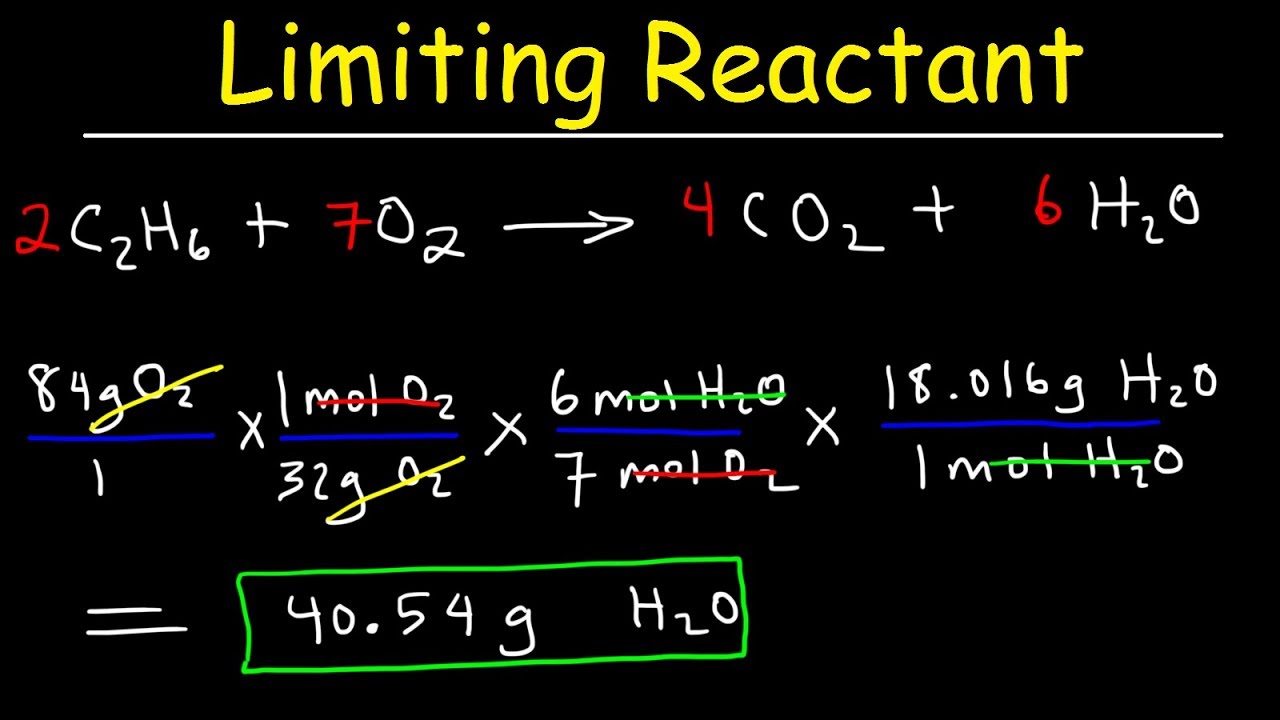
Limiting Reactant Practice Problems Youtube

Limiting Reagent Reactant Definition Examples And Problems

Chem 101 Dimensional Analysis Limiting Reagent Theoretical Yield Percent Yield Excess Reactant 2 Youtube

How To Identify The Limiting Reactant In A Drawing Of A Mixture Chemistry Study Com
No comments for "How to Determine Limiting Reactant"
Post a Comment